Pharma and Nutraceuticals: workflow and repository for packaging
Tailor-made solution for pharma and nutraceutical packaging, with a centralised repository and workflow for pack production
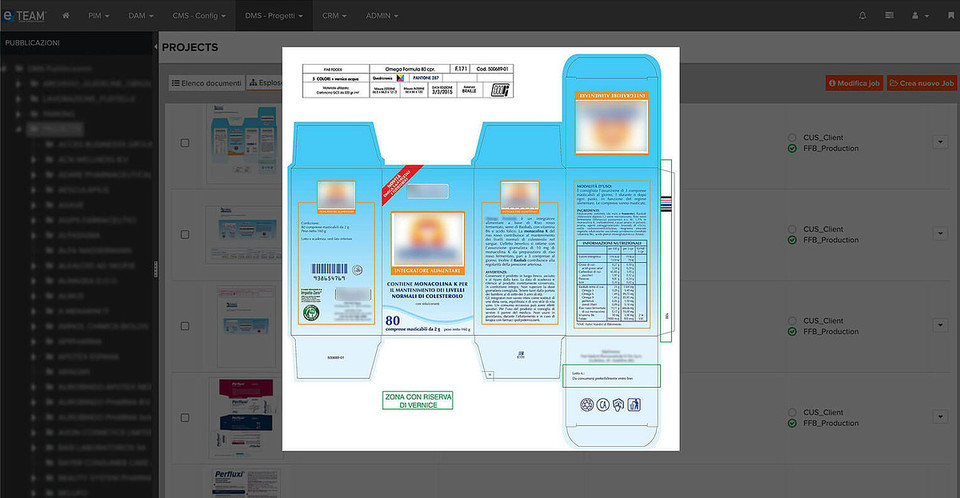
When it comes to packaging and labelling, pharmaceutical companies must adhere to strict laws and regulatory guidelines and pay close attention to artwork and labelling processes, tirelessly striving to get it right first time and taking every opportunity to mitigate sources of error.
The main causes of errors on pharmaceutical packaging have been identified as:
- Gaps and inconsistencies in the process
The artwork approval process is well managed, but upstream pack copy creation and approval is haphazard and leads to inaccurate content. - Lack of expertise
Employees involved in the process lack the necessary knowledge to carry out their tasks, which means that artwork reviewers are unsure of the areas they are required to check. - Inadequate decision-making process
Artwork is accelerated through a process with no checkpoints. Due to general mismanagement of the process and shortened deadlines they are riskily approved. - Ambiguity
In some cases too many people are potentially involved in an offline process and individual responsibility for certain actions, such as final approval, is never established. - Information errors
When preparing the artwork, the designer may not be sure that they will receive the final approved version of the package copy. This is common if uncontrolled or multiple sources of information are used, e.g. local copies of spreadsheets or even handwritten notes. - Human error
Assigning responsibility and oversight for upstream content and robust artwork verification and approval processes are important to ensure that any human error is detected before files are sent to press. - Technological errors
The packaging process necessarily involves multiple technologies (graphics, multiple business systems). If the technologies are not robust or controlled, some problems can occur such as copy and paste errors, problems with fonts, problems with PDF creation.
To solve these problems we have configured a specific solution for the Pharma and Nutraceutical sector.
How it works
- With eTEAM's Digital Asset Management, the company gains full control of the printing systems and executives. Artwork and labels are structured and sorted in the DAM. Access is secure and set according to permission levels per user.
- In the Product Information Management module, data is sorted by product sheets and translated for global markets in a unique way, ensuring data certainty from a single source of truth. There are automatic multilevel and multilingual ingredient list compositions for packaging and data sheets.
- A Project Management tool coordinates the packaging review and update process and the digital layout sharing and approval stages with graphic agencies.
The benefits
- elimination of paper or private exchanges by e-mail: all notes, indications and corrections are entered into the system
- more efficient management of jobs, saving time in sharing contents and validations
- drastically reduced margin of error with consequent savings in printing costs
- the creation flow of new artwork is faster
- the creation of a single central repository reduces errors and provides an archive of all jobs
Are you interested in this application?
Fill out the form below to have more informations about this application.
Required fields are marked with *


